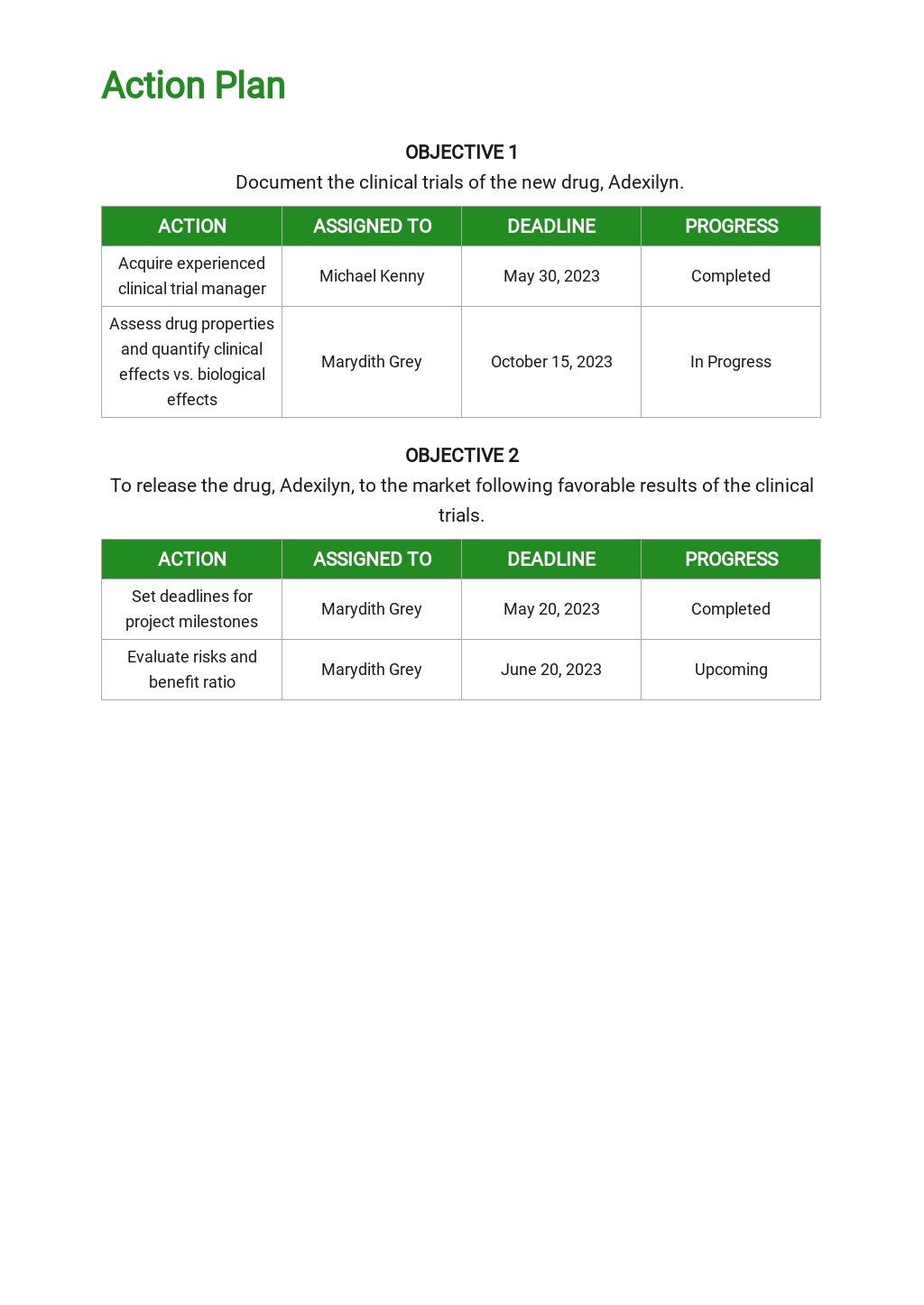
The clinical development plan (cdp) as per the medical device regulation (mdr) nhe. It should consider current knowledge about the condition being treated, the target patient population, and the regulatory landscape. Tailor a clinical development plan to the specific needs of the treatment being developed. The vision is transformed into distinct implementation phases and discrete steps, called clinical studies, each with well defined milestones and deliverables. Explore precision's custom clinical development planning, tailored to novel study needs.
The clinical development plan guide. Such a strategy encompasses various aspects of a program and can yield several benefits for a company, including: It should consider current knowledge about the condition being treated, the target patient population, and the regulatory landscape. Tailor a clinical development plan to the specific needs of the treatment being developed. In this task, the clinical trial concept will be developed based on the identified target disease indication and literature review findings.
A strategic, comprehensive clinical development plan (cdp) can help sponsors optimize efficiency, control costs, plan timelines, and maximize the probability of success for a new drug program. Tailor a clinical development plan to the specific needs of the treatment being developed. A clinical development plan — a comprehensive strategy for developing an investigational product through regulatory submission — is a critical component of drug development and helps ensure that new therapies are safe, effective, and of high quality. Explore precision's custom clinical development planning, tailored to novel study needs. The concept should outline the specific objectives, study design, patient population, and endpoints of the clinical trial.
Such a strategy encompasses various aspects of a program and can yield several benefits for a company, including: Tailor a clinical development plan to the specific needs of the treatment being developed. A clinical development plan — a comprehensive strategy for developing an investigational product through regulatory submission — is a critical component of drug development and helps ensure that new therapies are safe, effective, and of high quality. The clinical development plan (cdp) as per the medical device regulation (mdr) nhe. What is a clinical development strategic plan? In this task, the clinical trial concept will be developed based on the identified target disease indication and literature review findings. This article is intended to assist medical device manufacturers and medical writers to leverage the cdp as a tool to showcase their clinical evaluation strategy and plan. The clinical development plan guide. It should consider current knowledge about the condition being treated, the target patient population, and the regulatory landscape. There are two essential documents that any company developing a new pharmaceutical or biotechnology derived product requires to ensure it achieves its strategic goals. The vision is transformed into distinct implementation phases and discrete steps, called clinical studies, each with well defined milestones and deliverables. Advance global clinical development, with precision. The clinical development plan (cdp) is a new document that you must complete for the eu mdr technical documentation. A strategic, comprehensive clinical development plan (cdp) can help sponsors optimize efficiency, control costs, plan timelines, and maximize the probability of success for a new drug program. The concept should outline the specific objectives, study design, patient population, and endpoints of the clinical trial.
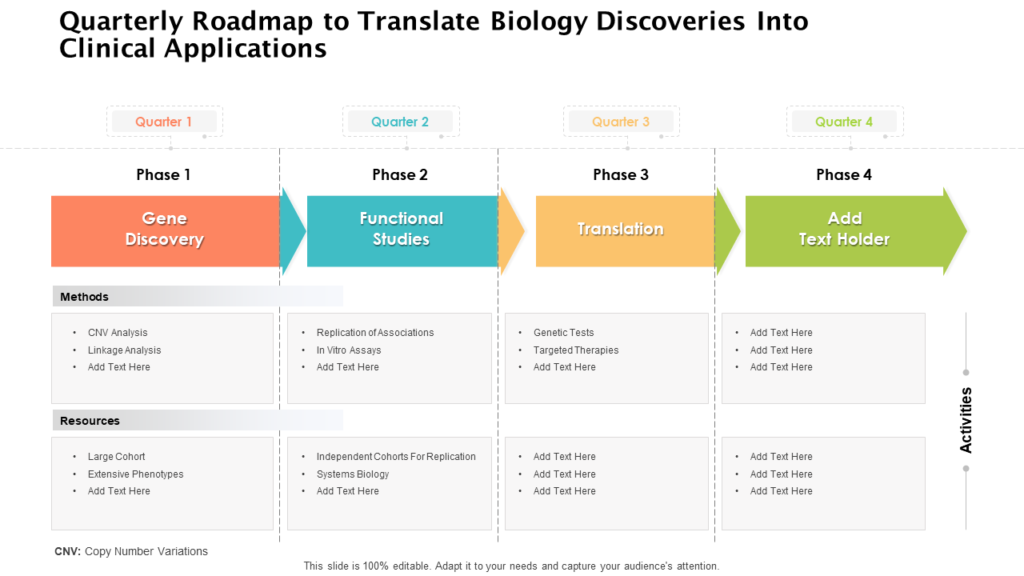
The Vision Is Transformed Into Distinct Implementation Phases And Discrete Steps, Called Clinical Studies, Each With Well Defined Milestones And Deliverables.
This article is intended to assist medical device manufacturers and medical writers to leverage the cdp as a tool to showcase their clinical evaluation strategy and plan. What is a clinical development strategic plan? Tailor a clinical development plan to the specific needs of the treatment being developed. It should consider current knowledge about the condition being treated, the target patient population, and the regulatory landscape.
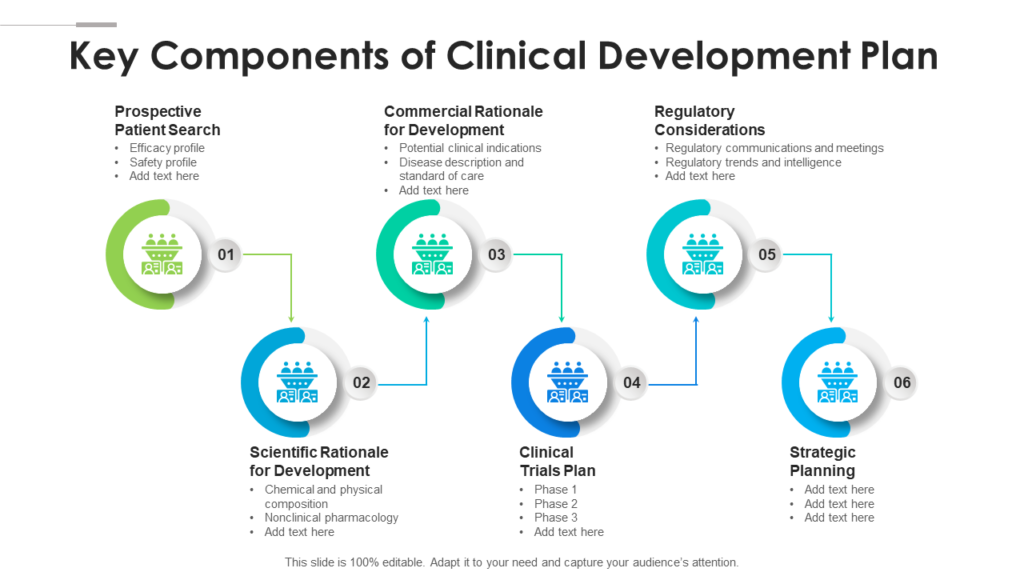
Such A Strategy Encompasses Various Aspects Of A Program And Can Yield Several Benefits For A Company, Including:
A clinical development plan — a comprehensive strategy for developing an investigational product through regulatory submission — is a critical component of drug development and helps ensure that new therapies are safe, effective, and of high quality. The clinical development plan guide. Explore precision's custom clinical development planning, tailored to novel study needs. A strategic, comprehensive clinical development plan (cdp) can help sponsors optimize efficiency, control costs, plan timelines, and maximize the probability of success for a new drug program.
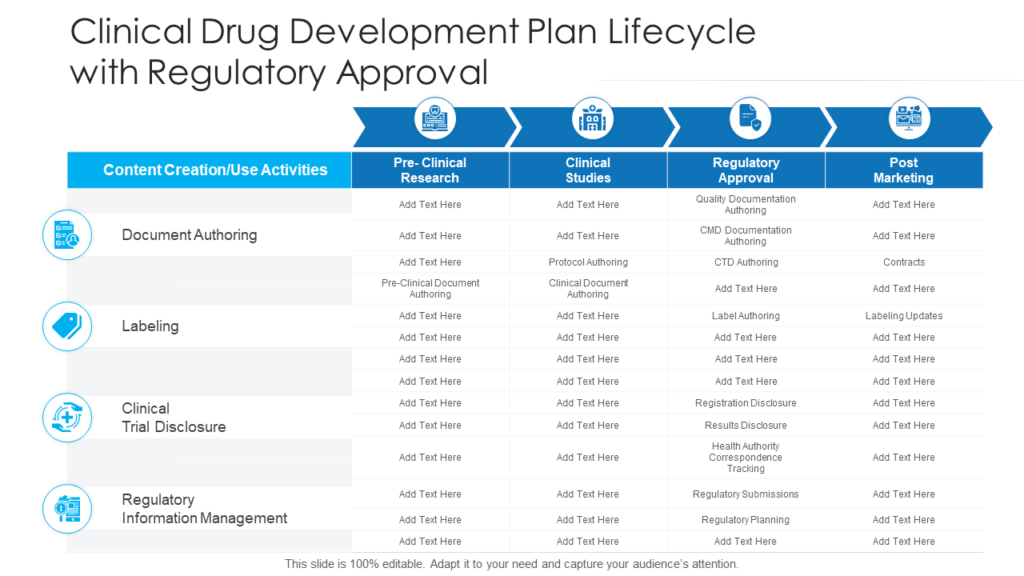
The Clinical Development Plan (Cdp) As Per The Medical Device Regulation (Mdr) Nhe.
The concept should outline the specific objectives, study design, patient population, and endpoints of the clinical trial. There are two essential documents that any company developing a new pharmaceutical or biotechnology derived product requires to ensure it achieves its strategic goals. In this task, the clinical trial concept will be developed based on the identified target disease indication and literature review findings. Advance global clinical development, with precision.