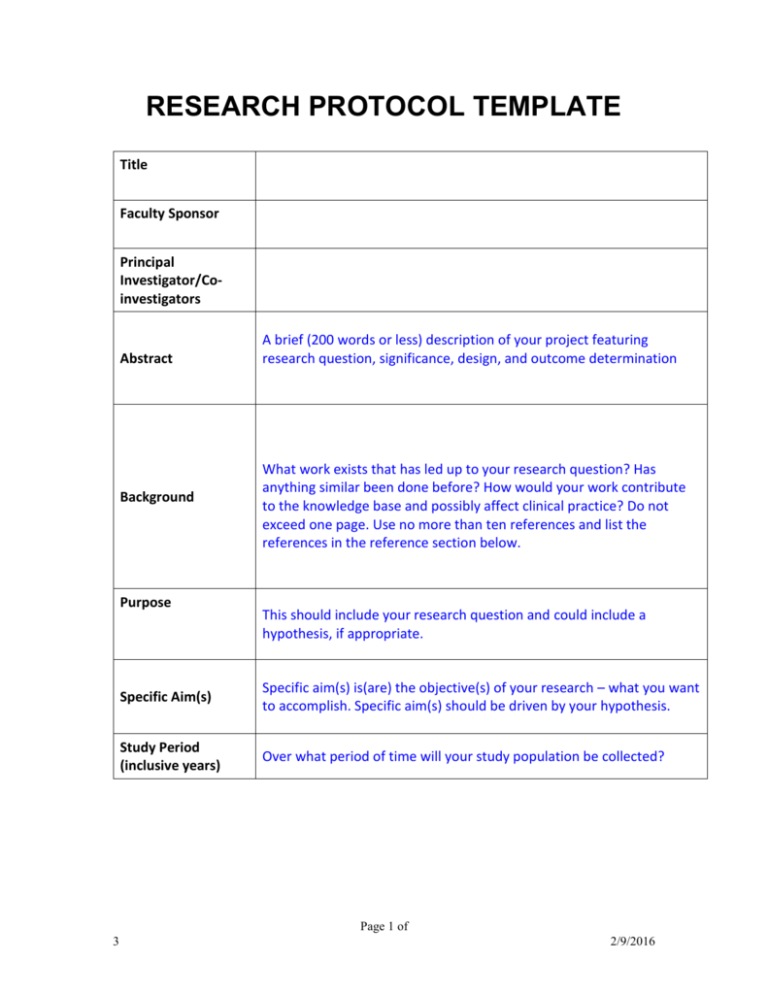
Web the transcelerate template introduces data standards in its endpoint sections and the use of controlled terminology permits automated reuse and introduces the concept of traceability. Web clinical content & reuse (cc&r) initiative, formerly known as common protocol template (cpt), aims to enhance clinical trial processes by developing common content for reuse across clinical trial documents in the clinical template suite (cts). Web in november 2018, transcelerate released its first version of a csr template. Web we would like to show you a description here but the site won’t allow us. Web within the next two years, ich is expected to issue a guideline, template and technical specification for a common electronic structured protocol, built in part on the foundations of transcelerate's cpt.
Web within the next two years, ich is expected to issue a guideline, template and technical specification for a common electronic structured protocol, built in part on the foundations of transcelerate's cpt. Web the transcelerate template introduces data standards in its endpoint sections and the use of controlled terminology permits automated reuse and introduces the concept of traceability. O participant is used rather than subject, healthy volunteer, or patient. Plus, resources to support their use, implementation, and adoption in clinical trials. Web in november 2018, transcelerate released its first version of a csr template.
Resources for our new common protocol template is now accessible via download. Web • the following terminology has been selected for use within transcelerate common templates (protocol, statistical analysis plan [sap], and csr) and is considered to be appropriate for all phases, study populations, and therapeutic areas. Web we would like to show you a description here but the site won’t allow us. Plus, resources to support their use, implementation, and adoption in clinical trials. Web within the next two years, ich is expected to issue a guideline, template and technical specification for a common electronic structured protocol, built in part on the foundations of transcelerate's cpt.
O participant is used rather than subject, healthy volunteer, or patient. Web clinical content & reuse (cc&r) initiative, formerly known as common protocol template (cpt), aims to enhance clinical trial processes by developing common content for reuse across clinical trial documents in the clinical template suite (cts). Web we would like to show you a description here but the site won’t allow us. Can be used individually or together to achieve content reuse. Web the transcelerate template introduces data standards in its endpoint sections and the use of controlled terminology permits automated reuse and introduces the concept of traceability. Web the first initiative is the creation of common clinical trial protocol templates, which will enable us, working with other stakeholders, to standardize the format of trial protocols and to. Web within the next two years, ich is expected to issue a guideline, template and technical specification for a common electronic structured protocol, built in part on the foundations of transcelerate's cpt. Web • the following terminology has been selected for use within transcelerate common templates (protocol, statistical analysis plan [sap], and csr) and is considered to be appropriate for all phases, study populations, and therapeutic areas. This csr template and associated resources, including a template for statistical analysis plans (saps), are located on the transcelerate website under the ‘common protocol template’ resources tab [ 16 ]. Plus, resources to support their use, implementation, and adoption in clinical trials. Resources for our new common protocol template is now accessible via download. Web templates for the common protocol (cpt), statistical analysis plan (sap), and clinical study report (csr) are available here. Web in november 2018, transcelerate released its first version of a csr template.
This Csr Template And Associated Resources, Including A Template For Statistical Analysis Plans (Saps), Are Located On The Transcelerate Website Under The ‘Common Protocol Template’ Resources Tab [ 16 ].
Can be used individually or together to achieve content reuse. Web within the next two years, ich is expected to issue a guideline, template and technical specification for a common electronic structured protocol, built in part on the foundations of transcelerate's cpt. Web the transcelerate template introduces data standards in its endpoint sections and the use of controlled terminology permits automated reuse and introduces the concept of traceability. Web in november 2018, transcelerate released its first version of a csr template.
Web The First Initiative Is The Creation Of Common Clinical Trial Protocol Templates, Which Will Enable Us, Working With Other Stakeholders, To Standardize The Format Of Trial Protocols And To.
Web • the following terminology has been selected for use within transcelerate common templates (protocol, statistical analysis plan [sap], and csr) and is considered to be appropriate for all phases, study populations, and therapeutic areas. O participant is used rather than subject, healthy volunteer, or patient. Resources for our new common protocol template is now accessible via download. Web we would like to show you a description here but the site won’t allow us.
Plus, Resources To Support Their Use, Implementation, And Adoption In Clinical Trials.
Web clinical content & reuse (cc&r) initiative, formerly known as common protocol template (cpt), aims to enhance clinical trial processes by developing common content for reuse across clinical trial documents in the clinical template suite (cts). Web templates for the common protocol (cpt), statistical analysis plan (sap), and clinical study report (csr) are available here.