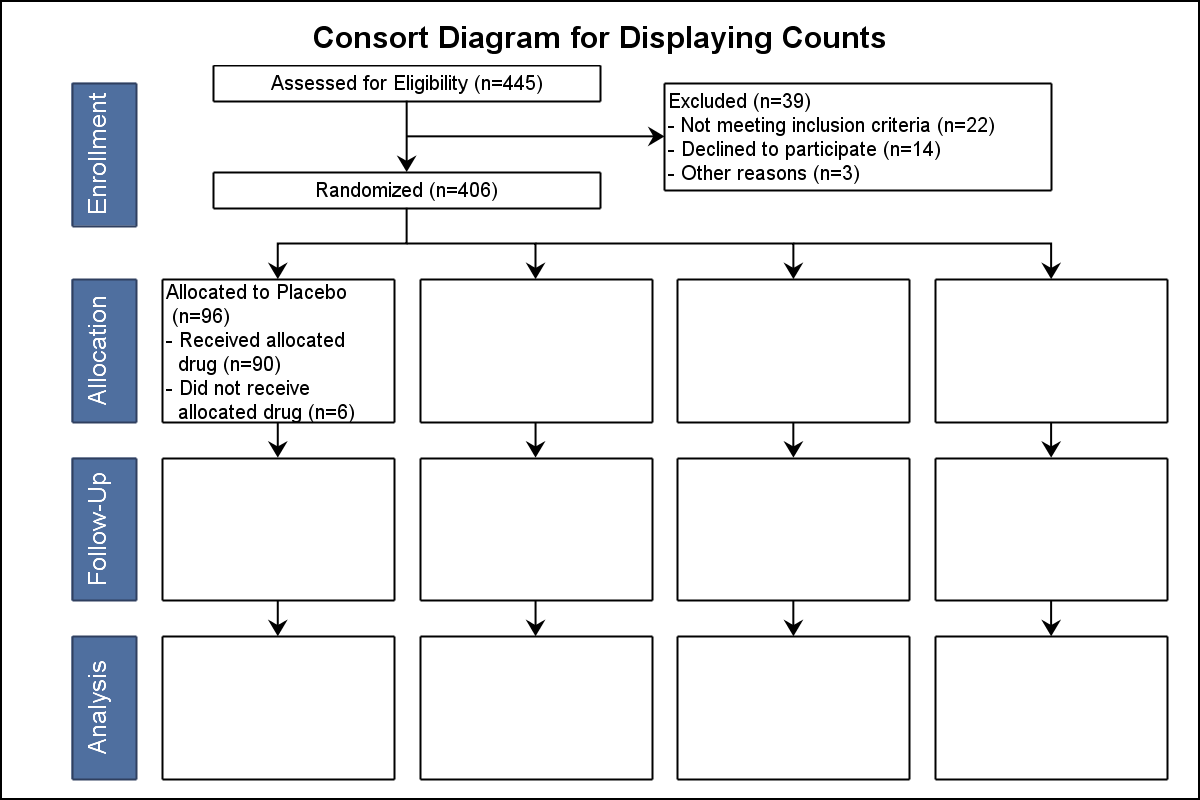
Web the expanded consort figure draws on theory, a prior meeting and recent recommendations for reporting factors related to external validity. Mary and tim created date: Mary and tim last modified by: The flow diagram can be accessed via the original published paper by following the pubmed links in the full bibliographic reference section of this web page. Web consort 2010 flow diagram assessed for eligibility (n= ) excluded (n= ) not meeting inclusion criteria (n= ) declined to participate (n= ) other reasons (n= )
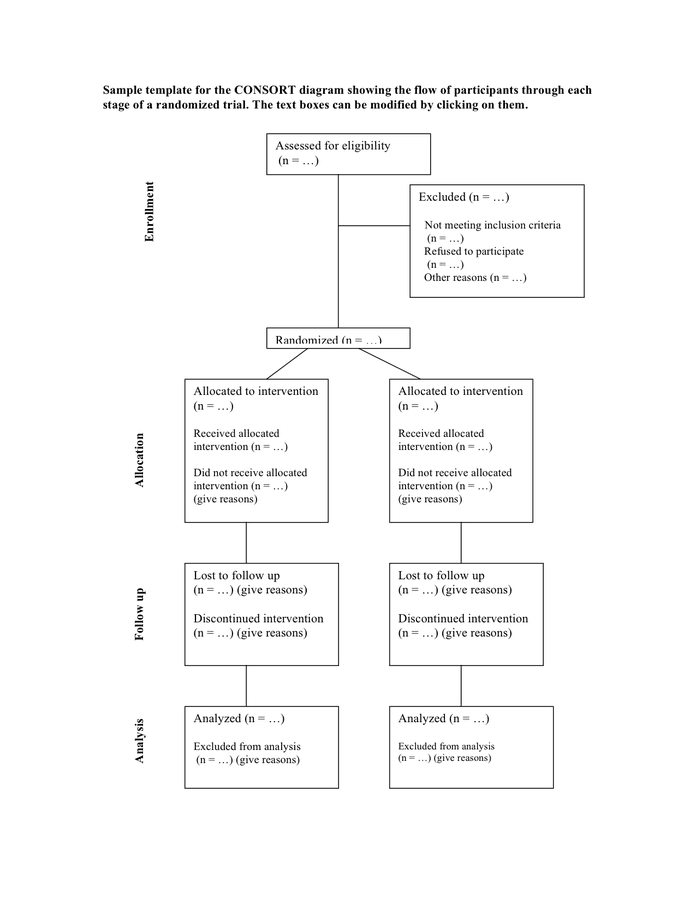
= give reasons n =. Web the consort statement (or simply consort) comprises a checklist of essential items that should be included in reports of rcts and a diagram for documenting the flow of participants through a trial. It expands the basic consort flow diagram for clinical trials to summarize external validity and contextual factors more concisely and transparently. Mary and tim created date: It is aimed at primary reports of rcts with two group, parallel designs.
Mary and tim last modified by: = give reasons n =. Web the consort statement (or simply consort) comprises a checklist of essential items that should be included in reports of rcts and a diagram for documenting the flow of participants through a trial. Web consort 2010 flow diagram assessed for eligibility (n= ) excluded (n= ) not meeting inclusion criteria (n= ) declined to participate (n= ) other reasons (n= ) Analysed n = excluded from analysis n = give reasons n =.
Mary and tim created date: Web the consort statement (or simply consort) comprises a checklist of essential items that should be included in reports of rcts and a diagram for documenting the flow of participants through a trial. Web the expanded consort figure draws on theory, a prior meeting and recent recommendations for reporting factors related to external validity. It expands the basic consort flow diagram for clinical trials to summarize external validity and contextual factors more concisely and transparently. Analysed n = excluded from analysis n = give reasons n =. Allocated to intervention n = received intervention n = did not receive intervention n = give reasons n =. = give reasons n =. It is aimed at primary reports of rcts with two group, parallel designs. Mary and tim last modified by: Web consort 2010 flow diagram assessed for eligibility (n= ) excluded (n= ) not meeting inclusion criteria (n= ) declined to participate (n= ) other reasons (n= ) The flow diagram can be accessed via the original published paper by following the pubmed links in the full bibliographic reference section of this web page.
The Flow Diagram Can Be Accessed Via The Original Published Paper By Following The Pubmed Links In The Full Bibliographic Reference Section Of This Web Page.
Mary and tim last modified by: Web consort 2010 flow diagram assessed for eligibility (n= ) excluded (n= ) not meeting inclusion criteria (n= ) declined to participate (n= ) other reasons (n= ) Analysed n = excluded from analysis n = give reasons n =. Web the consort statement (or simply consort) comprises a checklist of essential items that should be included in reports of rcts and a diagram for documenting the flow of participants through a trial.
It Is Aimed At Primary Reports Of Rcts With Two Group, Parallel Designs.
Mary and tim created date: = give reasons n =. Allocated to intervention n = received intervention n = did not receive intervention n = give reasons n =. It expands the basic consort flow diagram for clinical trials to summarize external validity and contextual factors more concisely and transparently.