Web nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research: Efs mcta (pdf) the authorship team acknowledged differences. Web master template agreement use can significantly speed the completion of contractual negotiations for a clinical trial. Web whereas, the institution and company have agreed to use the agreement, to accelerate the process of translating laboratory discoveries into treatments for patients, to engage. 1.1 compliance with laws, regulations, and good clinical practices.
Web find out how to use unmodified model agreements for clinical trials and clinical investigations in the uk, negotiated with english law and governance arrangements. Web nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research: Web learn how to initiate, review, negotiate, and execute clinical trial agreements (cta) with sponsors at the university of utah. The templates below have been shared by other groups, and are free to use and adapt for your research studies. This agreement is entered into on _______________________by and between.
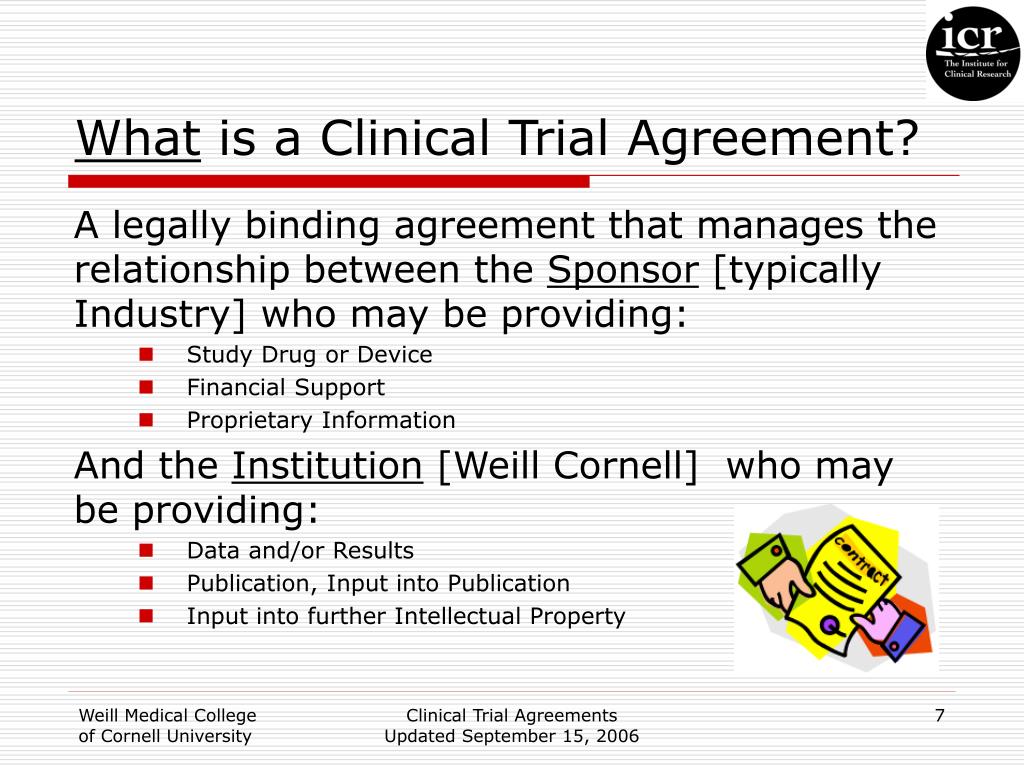
Efs mcta (pdf) the authorship team acknowledged differences. Institution is an organisation engaged in the diagnosis, treatment and prevention of disease and/or clinical research for the improvement of healthcare, and. Web a clinical trial agreement (cta) governs the relationship between the trial sponsor, who provides the device or drug to be studied along with the financial support. Web the early feasibility study master clinical trial agreement (mcta)* can be found here: This template is not to be used for.
Web this agreement describes the entire agreement between the parties concerning the subject matter hereof and supersedes all prior or contemporaneous agreements,. Phase 2 or 3 clinical trials that. Web welcome to global health trials' tools and templates library. Web find out how to use unmodified model agreements for clinical trials and clinical investigations in the uk, negotiated with english law and governance arrangements. Web nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research: Efs mcta (pdf) the authorship team acknowledged differences. Web a clinical trial agreement (cta) is a legally binding agreement that manages the relationship between the sponsor that may be providing the study drug or device, the. Learn what a cta is, when to use one, and what it should cover. Web welcome to global health trials' tools and templates library. Web a clinical trial agreement (cta) governs the relationship between the trial sponsor, who provides the device or drug to be studied along with the financial support. Web download free clinical trial templates for your clinical research, available in sharepoint, word, excel, and microsoft project formats. This agreement is entered into on _______________________by and between. Master agreements tend to require intense negotiations. Web group, and the parties agree to the use this standard agreement to accelerate the process of translating laboratory discoveries into treatments for patients, to engage communities. Web the acta is a neutral, streamlined clinical trial agreement template that is broadly accepted by both industry sponsors and institutions.
Web This Agreement Describes The Entire Agreement Between The Parties Concerning The Subject Matter Hereof And Supersedes All Prior Or Contemporaneous Agreements,.
It was developed by a. Find definitions, templates, guidelines, and contact. Phase 2 or 3 clinical trials that. Fast, easy & secureedit on any device5 star ratedform search engine
Web Find Out How To Use Unmodified Model Agreements For Clinical Trials And Clinical Investigations In The Uk, Negotiated With English Law And Governance Arrangements.
Web a clinical trial agreement (cta), clinical study agreement or clinical research agreement are all names for an agreement or contract between the university and. Web download a free clinical trial agreement template to agree terms between sponsors and institutions in 2023. Master agreements tend to require intense negotiations. 1.1 compliance with laws, regulations, and good clinical practices.
Web Nih Applicants Can Use A Template With Instructional And Sample Text To Help Write Clinical Protocols For The Following Types Of Research:
Web master template agreement use can significantly speed the completion of contractual negotiations for a clinical trial. Web group, and the parties agree to the use this standard agreement to accelerate the process of translating laboratory discoveries into treatments for patients, to engage communities. Web whereas, the institution and company have agreed to use the agreement, to accelerate the process of translating laboratory discoveries into treatments for patients, to engage. Web download free clinical trial templates for your clinical research, available in sharepoint, word, excel, and microsoft project formats.
Web A Clinical Trial Agreement (Cta) Governs The Relationship Between The Trial Sponsor, Who Provides The Device Or Drug To Be Studied Along With The Financial Support.
Please note that this page has been updated for 2015 following a quality check and review of the templates, and. This agreement is entered into on _______________________by and between. Institution agrees that institution, its investigator and study staff shall. Efs mcta (pdf) the authorship team acknowledged differences.