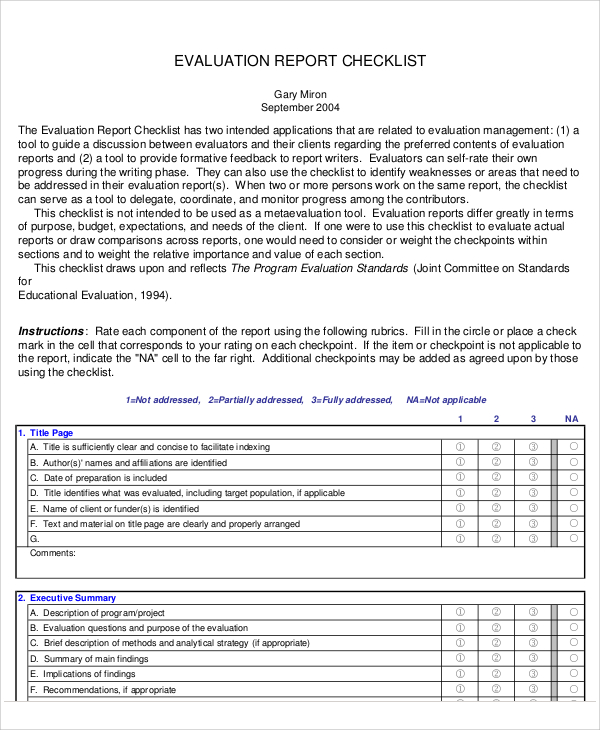
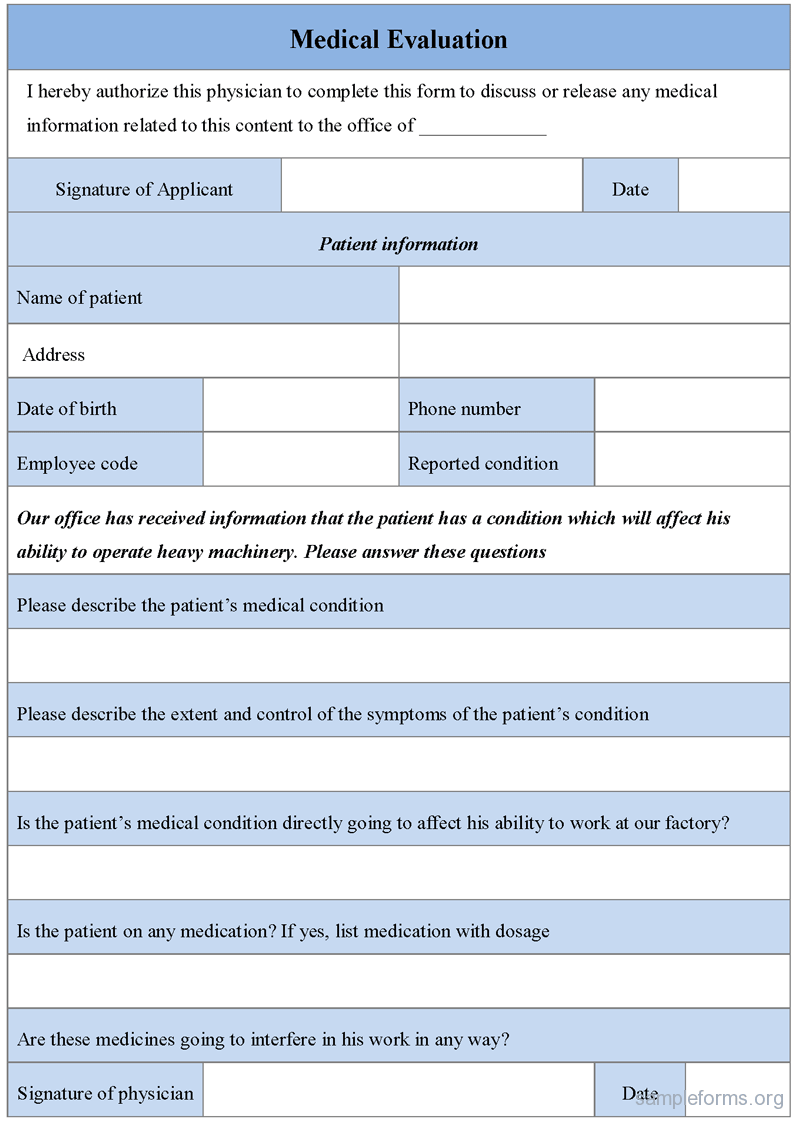
Web clinical evaluation assessment report template. It assesses the clinical risks associated with the. Web a clinical evaluation report (cer) is a comprehensive document that summarizes the results of the clinical evaluation process for a medical device. This page provides a range of documents to assist stakeholders in. It assesses the clinical risks associated with the use of a device, its.
Web in this guide: 6.1 what is clinical evaluation? A guide for manufacturers and notified bodies. Based on the findings in the clinical data review as well as in the risk analysis, it can be inferred that the probability of a patient. Web a clinical evaluation report (cer) is a comprehensive document that summarizes the results of the clinical evaluation process for a medical device.
Based on the findings in the clinical data review as well as in the risk analysis, it can be inferred that the probability of a patient. Stages in the clinical evaluation process. Web a clinical evaluation report (cer) documents the clinical evidence and conclusions for a medical device. Find out what makes cer challenging, how to use a template effectively,. Web download the template for clinical evaluation assessment report, a document required by eu medical device regulation.
Stages in the clinical evaluation process. Web in this guide: • evaluating data in terms of its suitability for establishing the safety and performance of the device. Check out our template walkthrough videos in which sebastian walks you through everything you need to know. Find out what makes cer challenging, how to use a template effectively,. Learn the steps, sources, elements and updates of a cer for the eu. Defining the scope and drafting a plan. Web how to use this template. It assesses the clinical risks associated with the use of a device, its. It assesses the clinical risks associated with the use of a device, its. The initial clinical evaluation is the crucial step in effective mental health treatment. This page provides a range of documents to assist stakeholders in. Web a clinical evaluation report (cer) provides clinical data to support a device’s clinical safety and efficacy. Making sure cer evaluators are qualified. Cancel anytime24/7 tech supportedit on any devicefree mobile app
This Page Provides A Range Of Documents To Assist Stakeholders In.
Based on the findings in the clinical data review as well as in the risk analysis, it can be inferred that the probability of a patient. Find out what makes cer challenging, how to use a template effectively,. Web • identifying available clinical data relevant to the device and its intended use. Learn how to write a clinical evaluation report (cer) according to meddev and mdr regulations for selling medical devices in the european union.
General Principles Of Clinical Evaluation.
Web a clinical evaluation report (cer) documents the clinical evidence and conclusions for a medical device. Web the clinical evaluation report (cer) provides clinical data to support the clinical safety and efficacy of a device. Web got stuck in your mdr clinical evaluation? The initial clinical evaluation is the crucial step in effective mental health treatment.
6.1 What Is Clinical Evaluation?
Defining the scope and drafting a plan. This document has been endorsed by the medical device coordination group (mdcg) established by article. Web how to use this template. Web a clinical evaluation report (cer) provides clinical data to support a device’s clinical safety and efficacy.
When Certifying Software As A Medical Device (Or Any Medical Device, Really), You Need A.
Stages in the clinical evaluation process. Web a clinical evaluation report (cer) provides clinical data to support a device’s clinical safety and efficacy. Learn the steps, sources, elements and updates of a cer for the eu. Web a clinical evaluation report (cer) is a comprehensive document that summarizes the results of the clinical evaluation process for a medical device.